Catalysis research with the X-ray microscope at BESSY II
Scientists studied changes in catalysts during the synthesis of ammonia from waste nitrates
Contrary to what we learned at school, some catalysts do change during the reaction: for example, certain electrocatalysts can change their structure and composition during the reaction when an electric field is applied. The X-ray microscope TXM at BESSY II in Berlin is a unique tool for studying such changes in detail. The results help to develop innovative catalysts for a wide range of applications. One example was recently published in Nature Materials. It involved the synthesis of ammonia from waste nitrates.
Ammonia (NH3) is a basic component of fertilisers and is critical to agricultural productivity around the world. Until now, ammonia has been synthesised industrially using the Haber-Bosch process, which is energy intensive and produces significant amounts of greenhouse gases that drive climate change. With the development of alternative methods, ammonia could be produced with significantly lower greenhouse gas emissions.
Better catalysts reduce emissions for Ammonia production
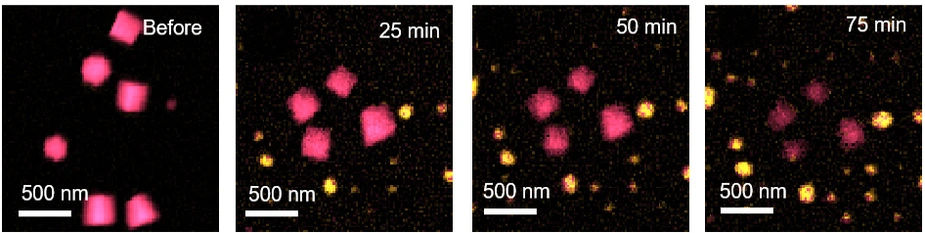
There are some promising approaches. For example, a team at the Fritz Haber Institute has been investigating a catalyst based on nanocrystalline copper oxide. During the catalytic reaction, an increasing proportion of these nanocrystals transformed into metallic particles of pure copper. The morphological changes were documented under the transmission electron microscope (TEM), but to gain insights into the chemical processes during the reaction, the FHI team collaborated with the group of Prof. Gerd Schneider at HZB.
Unique insights at the TXM
The transmission X-ray microscope (TXM) is the only one of its kind in the world for catalysis research, as catalysts can be examined in both the TEM and the TXM in the same specimen holder to obtain complementary information on catalysis. As an operando microscope, the TXM enables spectroscopic data to be obtained at the nanoscale, allowing chemical processes and reactions to be analysed.
'We were able to show that both copper dioxide and metallic copper particles exist for long periods of time and are kinetically stabilised by certain surface hydroxide groups,' says HZB physicist Dr. Christoph Pratsch from Schneider's team, who carried out the TXM investigations.
Crucial interactions examined
The composition of this mixture and the form of the resulting catalysts depend strongly on the applied electrical potential, the chemical environment and the duration of the reaction. The interaction between the electrolyte and the catalyst is crucial for the yield of ammonia and thus for the efficiency of the desired reaction.
Two new X-ray microscopes for future experiments
The X-ray microscopy team is currently developing two new microscopes. A new TXM will allow routine spectromicroscopic investigations from the soft to the hard X-ray range, including the use of phase rotations of the X-ray waves in the object. 'We will be able to distinguish between processes inside and on the surface of catalysts by measuring the electron emission,' explains Gerd Schneider. In addition, the distribution of elements in nanoscale catalysts can be measured using X-ray fluorescence. The new microscopes can already be used at BESSY II. However, their full potential will be unleashed at the successor facility BESSY III, which is scheduled to go into operation in 2035. The two new instruments will then provide even deeper insights into catalytic processes.
Further information:
Press release of Fritz-Haber-Institute on the “Secret life of catalysts”
Publication:
Nature materials (2025): Revealing catalyst restructuring and composition during nitrate electroreduction through correlated operando microscopy and spectroscopy
Aram Yoon, Lichen Bai, Fengli Yang, Federico Franco, Chao Zhan, Martina Rüscher, Janis Timoshenko, Christoph Pratsch, Stephan Werner, Hyo Sang Jeon, Mariana Cecilio de Oliveira Monteiro, See Wee Chee & Beatriz Roldan Cuenya
DOI: 10.1038/s41563-024-02084-8
Contact:
Helmholtz-Zentrum Berlin für Materialien und Energie
Department X-Ray Microscopy
Dr. Christoph Pratsch
+49 30 8062-13177
christoph.pratsch(at)helmholtz-berlin.de
Dr. Stephan Werner
+49 30 8062-13181
stephan.werner(at)helmholtz-berlin.de
Prof. Dr. Gerd Schneider
+49 30 8062-13131
gerd.schneider(at)helmholtz-berlin.de
Press release HZB, 27 March 2025